Ringworm is an infection of skin, hair, or claws caused by a type of fungus. In dogs, about 70% of ringworm cases are caused by the fungus Microsporum canis, 20% by Microsporum gypseum, and 10% by Trichophyton mentagrophytes. In young or debilitated animals and in Yorkshire Terriers, infection may be persistent and widespread. The infecting fungus is spread easily in the environment. People can easily be infected with these fungi.
Most cases of ringworm are spread by contact with infected animals or contaminated objects such as furniture or grooming tools. Broken hairs with associated spores are important sources for spread of the disease. Contact does not always result in infection. Whether infection is established depends on the fungal species and on host factors, including age, health, condition of exposed skin surfaces, grooming behavior, and nutrition. Infection leads to short-lived resistance to reinfection. Under most circumstances, dermatophytes grow only in the dead cells of skin and hair, and infection stops on reaching living cells or inflamed tissue. As inflammation and host immunity develop, further spread of infection stops, but this process may take several weeks.

Infected dogs develop bald, scaly patches with broken hairs. Dogs may also develop acne-like bumps on the skin. The most common sites affected by ringworm are the face, ear tips, tail, and feet. Ringworm is diagnosed by fungal culture, examination with an ultraviolet lamp, and direct microscopic examination of hair or skin scale. Fungal culture of hairs and scrapings from the affected areas is the most accurate method. Direct microscopic examination of hairs or skin scrapings may allow early diagnosis.
Ringworm infections in healthy adults may clear up without treatment, but treatment might speed recovery and decrease the spread of the fungus in the environment. Medicated shampoos and dips are usually used to treat the entire coat. Diluted bleach can be used to clean the pet’s environment. Additional, oral medications are needed in longterm or severe cases and are often necessary for Yorkshire Terriers. Your veterinarian can provide you with information about any treatment that may be appropriate for your pet and advise you regarding precautions you should take to avoid ringworm infection in yourself and members of your family.

Dermatophytoses in Dogs and Cats
Dermatophytoses have a variety of clinical presentations, and a step-by-step logical approach is crucial for proper diagnosis.

ermatophytes are pathogenic fungi that cause skin disease in small animals and humans; they can represent a significant problem for shelter animals and household pets allowed extensive exposure to an outdoor environment. The most common dermatophyte infections diagnosed in small animals are caused by Microsporum canis, Microsporum gypseum, and Trichophyton mentagrophytes, and the most common sources are cats (M canis), infected soil (M gypseum), and rodents (T mentagrophytes).1
Although dermatophytes are transmitted by contact with arthrospores, exposure to arthrospores does not always lead to establishment of an infection. Resistance to infection is associated with a strong cell-mediated response.2 Infection is more likely to develop in very young, geriatric, or stressed animals.3 In shelter cats, infections with dermatophytes other than M canis are rare and do not seem to represent a treatment challenge; most cats reach mycological cure within 3 weeks of treatment.4
Clinical Signs and Diagnoses
Dermatophytoses have a variety of clinical presentations. Because dermatophytes are keratinophilic, their targets are hairs and nails.
Folliculitis
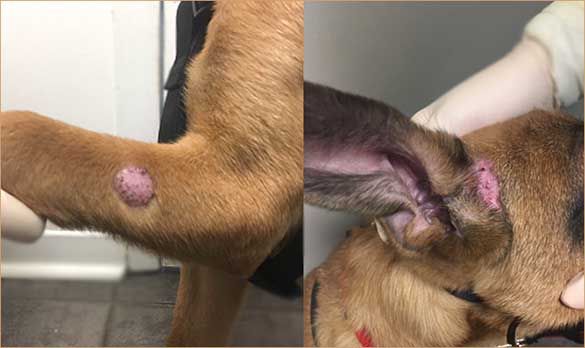
When dermatophytes affect the hairs, they lead to folliculitis (FIGURE 1). Infections appear as papules and pustules (FIGURE 2), which rapidly rupture, leaving epidermal collarettes, circular areas of alopecia (FIGURE 3), and crusts. Differential diagnoses for folliculitis include staphylococcal infection (FIGURE 4) and, particularly in dogs, demodicosis (FIGURE 5). Thus, when assessing a dog with signs of folliculitis, perform cytologic examination to look for intracellular bacteria and deep skin scrapings to rule out demodicosis.




In the author’s experience, bacterial folliculitis is typically more common than dermatophyte infection in dogs; however, do not make assumptions and skip diagnostic steps. Dermatophytosis can represent a significant challenge in dogs of certain breeds, such as Yorkshire terriers. It is very important to go through the list of differential diagnoses and work up each case in a step-by-step logical manner. Other causes of pustular disease (e.g., pemphigus foliaceus) that are not oriented to follicles should also be considered when working up patients presented for hair loss and crusting. A good initial approach is ruling out causes of folliculitis first by doing cytology, deep skin scrapings, and fungal culture.
Nodular Lesions
Sometimes dermatophytes are accidentally inoculated into the dermis (e.g., during injury), which leads to a severe inflammatory response and development of a nodular lesion called a kerion.5 This nodular lesion is typically found on the bridge of the nose of dogs that like to dig. Even after the dermatophytes die, the severe inflammatory response can persist. Another manifestation of nodular disease caused by dermatophytes is the dermatophytic mycetoma, also called pseudomycetoma. This uncommon dermal/subcutaneous infection is primarily found on Persian cats and appears as nodules with draining tracts, typically on the back. These cats are usually presented because they do not respond to antibiotic therapy.
For patients with nodular lesions, it is important to determine whether they result from infectious agents. Biopsy for histopathology and culture is frequently required, but cytology should also be performed since impression smears can be diagnostic.5
Nail Lesions
Dermatophytes can affect the footpads (FIGURE 6) and nails (FIGURES 7 AND 8). Dermatophyte-infected nails become brittle and appear deformed, especially in dogs with M gypseum infection.6
Differential diagnoses for deformed and brittle nails can include systemic lupoid onychodystrophy or genetically inherited dystrophies that are not immune mediated. When working up these cases, before considering biopsies or other invasive diagnostic tests, collect samples of the affected nails (e.g., nail clippings) and submit them for fungal culture.


Diagnostic Techniques
A recently published clinical consensus paper on dermatophytosis in small animals concluded, after review of the current literature, that no single diagnostic test can be identified as the gold standard for the diagnosis of dermatophytosis.1 The authors state that diagnosis is made by using complementary methods to indicate an active infection, such as direct examination of the arthrospores on infected hairs and fungal culture of hairs collected with a sterile toothbrush.
Wood’s Lamp
Use of a Wood’s lamp is still recommended as a screening tool, and it is now accepted that most M canis infections will fluoresce apple green under a Wood’s lamp. Depending on the study, positivity ranges from 91% to 100%; the higher percentages were found in studies of experimentally induced infection.1 The ability to fluoresce develops after the first week of infection and can persist at the tip of the hairs after resolution of the infection.1 The authors of the consensus paper concluded that the Wood’s lamp is a better tool for initial screening than for monitoring the success of treatment.1 Clinicians need to be familiar with how to properly use a Wood’s lamp. Examination should start at the patient’s head, moving slowly back while holding the lamp close to the skin (2 to 4 cm), distinguishing the apple green fluorescence of hairs with dermatophytosis from the false blue fluorescence that can be seen with scaling and some topical products.1
Fungal Culture
For decades, the gold standard of dermatophyte diagnosis has been fungal culture. However, the reality is that this approach simply indicates the presence or absence of spores on the hairs. The success of this approach depends partly on the sampling technique and the area that was selected for culture. A technique widely reported in the literature is the toothbrush approach, which may detect asymptomatic carrier animals. In 2017, Di Mattia et al. reported the importance of properly inoculating the samples onto the fungal culture media. Pressing the toothbrush onto the plate is the optimum way to maximize M canis growth and minimize contaminant growth.7
Direct Examination
Plucking hairs from the margins of an existing lesion is considered useful for specifically addressing the question of whether the lesion may be caused by a dermatophyte, although hair plucks can still lead to negative results.1 Slides with 10 to 20 plucked hairs in mineral oil can be microscopically examined for arthrospores.
Polymerase Chain Reaction
In recent years, use of polymerase chain reaction (PCR) to diagnose dermatophytosis has drawn attention, and results of studies differ, depending on the PCR used. In 2018, Moriello et al. found that quantitative PCR (qPCR) testing of toothbrush fungal culture samples of lesions on shelter cats was a reliable test for confirming disease.8 qPCR and fungal culture results matched in 94% of cases. qPCR also correctly identified 2 cats as not infected. Mycological cure was correctly identified for 65.2% of cats by Microsporum species qPCR assays and 84.8% by M canis assays.

Cafarchia et al. reported high accuracy of one step-PCR for dogs (area under the curve [AUC] >90) but only moderate accuracy for cats (AUC = 78.6).9 In the same study, a nested PCR was accurate (AUC = 93.6) for samples from cats and achieved higher specificity (94.1% and 94.4%) and sensitivity (100% and 94.9%) for samples from dogs and cats, respectively. Another study that compared the performance of nested PCR with direct microscopy and culture found the degree of agreement to be higher for nested PCR and direct microscopy (94.4%) than for culture (83.3%).10 Similar to fungal cultures, a positive PCR does not confirm active infection, and results need to be interpreted in the context of the clinical signs. A recent study also reported that PCR is less sensitive than previously reported but that it is more specific, thus increasing the risk for false-negative results.11
Treatments
Successful treatment of dermatophytosis in dogs and cats requires the combination of topical treatment, systemic treatment, and environmental disinfection.
Topical Treatments
Because dermatophytosis is transmitted by contact with arthrospores, topical therapy is an essential component of treatment. Topical treatment helps speed the resolution of infection and decrease the shedding of arthrospores into the environment.
.

Clipping
For decades, clipping has been a necessary part of dermatophytosis treatment; however, clipping is currently being reconsidered because whole-body clipping is stressful and the common microtrauma of the skin can worsen the infection. Thus, whether to clip should be decided on a case-by-case basis; clipping is not necessary for short-coated animals.1
Dips, Shampoos, and Rinses
In the United States, lime sulfur dips are still recommended. Several studies have documented the efficacy of lime sulfur dips, and twice weekly application is more effective than once weekly.1,12-14 The dip has residual activity on the coat, whereas that of shampoos is shorter.13 In the author’s experience, common side effects of lime sulfur are dryness and yellow discoloration. Although older studies reported on the potential risk for oral ulcers in cats that lick their coat while still wet, newer studies have not confirmed these findings, raising the possibility that the older studies used more concentrated solutions.1 Most current lime sulfur formulations for veterinary use are 97.8% saturated lime sulfur, which is applied at a dilution of 8 oz/gallon of water.
When clients object to the smell of the sulfur dip, other options available in the United States include shampoos and rinses. Among the shampoos, neither chlorhexidine nor miconazole alone is considered an effective treatment. The most effective topical treatment is the combination of miconazole and chlorhexidine used twice weekly.15,16 Although clinicians commonly believe that chlorhexidine has antifungal properties, the efficacy of chlorhexidine for dermatophytosis has been shown to be poor.17
Topical enilconazole is also effective for treating dermatophytosis in small animals18 but is currently not available in the United States. In countries where enilconazole rinse is available, it is considered a very effective topical option against dermatophytosis.19 Although enilconazole is generally well tolerated, it has been reported to cause hypersalivation, muscle weakness, and slightly elevated serum alanine aminotransferase (ALT) concentrations in Persian cats.16
The efficacy of shampoos containing ketoconazole for animals has not been assessed by in vivo studies. In vitro studies support effectiveness of ketoconazole, but no clinical trial on ketoconazole shampoo for dermatophytosis in small animals has been published.19,20
Similarly, no in vivo studies support the use of topical climbazole for dermatophytosis in small animals. In vitro studies showed good residual activity of 0.5% climbazole with chlorhexidine.19
Only 1 study on topical terbinafine has been published, and it reported a good response.20
Systemic Treatments
The ideal choices for systemic therapy are drugs that are keratinophilic and lipophilic and accumulate in the skin and keratin. Currently, the most effective systemic treatments for both dogs and cats are oral itraconazole or oral terbinafine.1
Itraconazole has a long half-life in cats21 and a great propensity to accumulate in hairs and skin. This property enables use of pulse therapy, which decreases the cost of therapy; daily administration for 1 week followed by 1 week on and 1 week off has shown clinical success.22 Others have started the pulse regimen only after giving the drug daily for 4 weeks.23 The most commonly used dose for itraconazole in dogs and cats is 5 mg/kg once daily. Because itraconazole affects cytochrome P450, it is important to consider drug interactions and decrease doses of other medications if their metabolism is affected by this interaction (e.g., cyclosporine).
Compounded formulations of itraconazole should be avoided; several studies showed that compounded formulations had subtherapeutic values in treated animals.24,25 Generic itraconazole seems to be better, although therapeutic monitoring is helpful because concentration can be very variable.23 If treatment failure is noted, switch to the product formulated for humans, Sporanox (Janssen Pharmaceuticals, janssen.com). Note also that when used at higher doses, itraconazole has been reported to trigger vasculitis in dogs.26 Clinicians should consider the possibility of this adverse effect when using this medication, although the high doses are typically reserved for systemic mycosis and are not recommended for dermatophytosis. A liquid formulation (Itrafungol; Elanco, elanco.com) is available for use in cats. Reported adverse effects of itraconazole include elevated liver enzymes and anorexia in dogs and decreased food consumption, depression, and elevated serum ALT concentrations in cats.1
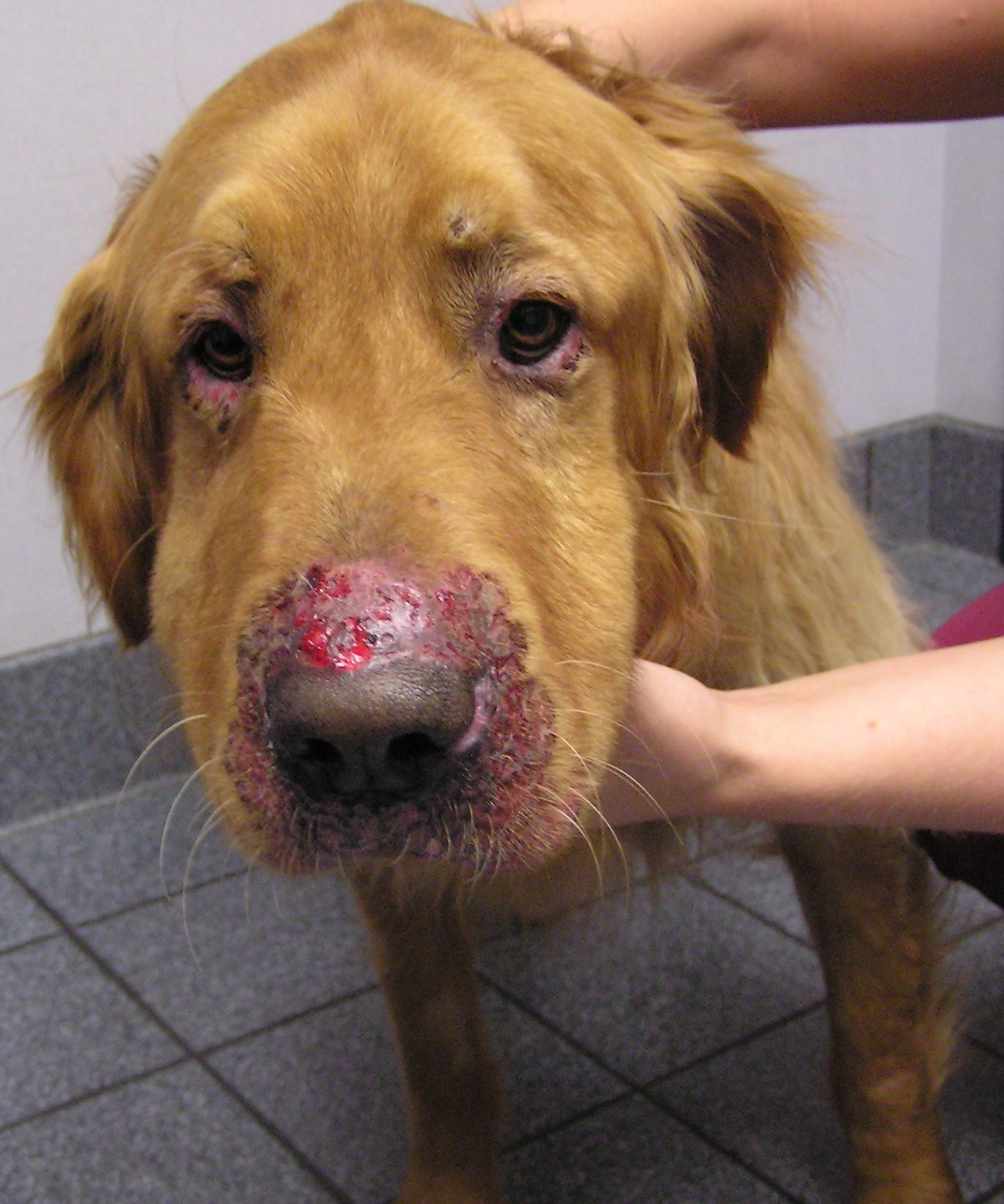
Terbinafine is very keratinophilic and accumulates in hairs,27 making it possible to do pulse therapy, which decreases cost and adverse effects. Terbinafine has excellent activity against dermatophytes, and one study has shown that it is efficacious and can represent a suitable and cheaper alternative for shelter cats.14 The commonly used dose range for terbinafine is 20 mg/kg14 to 40 mg/kg;28 efficacy is increased at higher doses. Although terbinafine does not have the same effect on cytochrome P450 as the azoles, its metabolism largely involves the liver; monitoring of liver values may be necessary when treatments are prolonged.
Ketoconazole is effective against dermatophytes, although it is not as good a treatment choice as itraconazole or terbinafine. Ketoconazole has been used in cats;29 however, because it is not typically well tolerated and frequently causes nausea and anorexia, it is best to reserve this medication for dogs. Ketoconazole is typically prescribed for dogs at 5 mg/kg PO q12h and is best administered with food to minimize adverse effects and increase absorption.
Fluconazole has poor activity against dermatophytes in vitro30 and is no longer recommended for treatment of dermatophytosis. Fluconazole is also water soluble and does not have the same ability as itraconazole and ketoconazole to accumulate in the skin and keratin.
Griseofulvin has historically been used to treat dermatophytosis but safer and more effective choices are now available. Thus, griseofulvin is rarely selected as a treatment.
Lufenuron was previously considered as a possible treatment, but studies have shown no efficacy.31,32 Therefore, lufenuron should not be considered as a treatment option.
Vaccines do not prevent development of dermatophytosis33 and thus should not be used for that purpose.
Environmental Decontamination
Environmental decontamination is a major part of dermatophytosis treatment. It also minimizes false-positive fungal culture results. Although separating animals for the purpose of minimizing contamination34 has been advocated for decades, confinement needs to be done with care because it can be very stressful, particularly for young animals.1 Thus, the duration of isolation should be minimized to what is needed to decontaminate the environment. The need for extended isolation can be decreased by weekly cleaning and use of topical therapy.
Studies have shown that weekly cleaning is very effective for removing infective arthrospores.35,36 The most important part of the decontamination process is the actual hard cleaning, which involves removal of debris and hairs. Cleaning can be accomplished with over-the-counter household detergents.37 Hard surfaces can be disinfected with 1:100 concentration household bleach or accelerated hydrogen peroxide.37,38 Machine washing of soft fabrics should be done by using the longest cycle, to maximize spore removal.
Monitoring Treatment Success
Clinical cure does not always equate to mycological cure. Thus, hair regrowth and the clinical appearance of the patient may not be sufficient criteria for decisions about duration of treatment. It is currently recommended that monitoring therapy and establishing whether a patient is completely cured should be based on a combination of resolution of clinical signs and a negative fungal culture.1 Extent of infection can be monitored by performing weekly cultures.1
Dead fungal organisms can still be detected by PCR. Thus, a positive PCR result along with resolution of signs may indicate that the patient still has some spores on the coat rather than an actual infection. Patients may, for example, pick up spores from a contaminated environment although they have developed immunity and are no longer actively infected. These animals may represent a source of infection for other individuals around them, and further environmental decontamination may be warranted. Fungal cultures are easily available in practice and can help with treatment monitoring. When in doubt, fungal culture can be repeated to ensure that the results are truly negative.

Long-haired cats have a propensity for subclinical dermatophytosis39 and therefore are potential carriers. When dealing with an outbreak of dermatophytosis in a multicat household, every cat should be cultured to determine which are truly negative and which are clinically normal but carrying arthrospores on their coats and potentially acting as a source of infection for others.40
Summary
Dermatophytosis is a zoonotic but curable disease. When examining patients with folliculitis, a step-by-step logical approach is crucial for proper diagnosis. Diagnosis can be obtained by a combination of clinical signs and positive fungal culture results. In the absence of clinical signs, positive PCR or culture results may simply indicate presence of arthrospores on the coat without active infection. Because dermatophytes are not part of the regular flora, the source of the arthrospores should be identified. Wood’s lamp examination is still recommended for fast screening of M canis infection. Most affected patients require a combination of topical and systemic therapies. The most effective oral medications are itraconazole and terbinafine, which can be combined with twice weekly topical lime sulfur dips and/or shampoos containing both miconazole and chlorhexidine. Establishing whether a patient is completely cured should be supported by a combination of resolution of clinical signs and negative culture.